StudentShare


Our website is a unique platform where students can share their papers in a matter of giving an example of the work to be done. If you find papers
matching your topic, you may use them only as an example of work. This is 100% legal. You may not submit downloaded papers as your own, that is cheating. Also you
should remember, that this work was alredy submitted once by a student who originally wrote it.
Login
Create an Account
The service is 100% legal
- Home
- Free Samples
- Premium Essays
- Editing Services
- Extra Tools
- Essay Writing Help
- About Us
✕
- Studentshare
- Subjects
- Health Sciences & Medicine
- Medical Devices Decontamination
Free
Medical Devices Decontamination - Assignment Example
Summary
In the paper “Medical Devices Decontamination” the author compares and contrasts the quality of water required for steam generation and for the final rinse stage of an endoscope washer-disinfector. Disinfection is a process essential for ensuring the effective removal of infecting organisms…
Download full paper File format: .doc, available for editing
GRAB THE BEST PAPER91.9% of users find it useful
- Subject: Health Sciences & Medicine
- Type: Assignment
- Level: Undergraduate
- Pages: 4 (1000 words)
- Downloads: 0
Extract of sample "Medical Devices Decontamination"
Medical devices decontamination - Compare and contrast the quality of water required for steam generation and for the final rinse stage of an endoscope washer-disinfector
INTRODUCTION:
Disinfection is a process essential for ensuring an effective removal of infecting organisms from medical devices such as endoscopes and dental apparatus etc which need to be re used again and again.
Disinfection is generally carried out by steam generation in autoclaves or by the use of an endoscope washer disinfector. For both of these equipments, water is required.
The autoclave requires water for steam generation and the endoscope washer-disinfectors require water during the final rinse stage after being treated with all the chemicals. The purpose is to remove the residues of the chemicals used which, if left untreated at this stage, can lead to damage to the equipment.
Autoclaves require the steam generation at high temperatures in order to carry out an effective disinfection. These higher temperatures are targeted to kill the micro organisms sensitive to heat.
The endoscope washer disinfectors usually consist of a series of steps in which disinfection is primarily carried out by different bactericidal chemicals and the final step is to rinse the equipment in clean water in order to get rid of the remnants of these chemicals as well which if not removed properly, can lead to a damage to the endoscope thereby limiting its efficiency.
If the quality of water used in these equipments is not maintained, it can lead to an outbreak of infections resulting from the micro organisms present in ordinary water.
Simple tap water cannot be used for this purpose as it can contaminate the endoscope with micro organisms, chemicals, particulates and endotoxins; thus transmitting infection to the patients. Thus only the sterile water is used for disinfecting endoscopes.
Now there are certain criteria and standards set for the quality or water to be used either for the steam generation in autoclaves, or for the final rinse stage of disinfection of endoscopes.
QUALITY OF WATER FOR STEAM GENERATION:
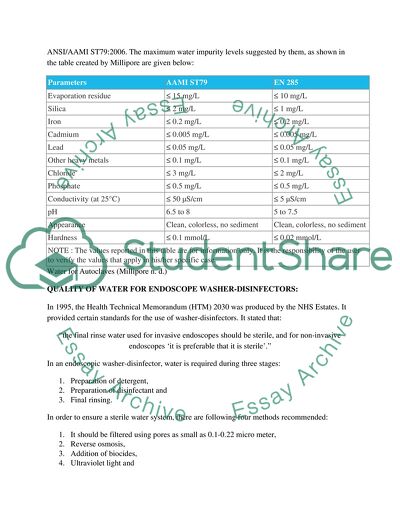
The water used for steam generation in autoclaves can also adversely affect the performance and life time efficacy of the autoclave. The water used in autoclaves must be free of ions, metals, particles, bacteria and chlorides. The water used for steam generation in autoclaves has been set various standards by both the European standard EN 285:2006 and the United States standard ANSI/AAMI ST79:2006. The maximum water impurity levels suggested by them, as shown in the table created by Millipore are given below:
Parameters
AAMI ST79
EN 285
Evaporation residue
≤ 15 mg/L
≤ 10 mg/L
Silica
≤ 2 mg/L
≤ 1 mg/L
Iron
≤ 0.2 mg/L
≤ 0.2 mg/L
Cadmium
≤ 0.005 mg/L
≤ 0.005 mg/L
Lead
≤ 0.05 mg/L
≤ 0.05 mg/L
Other heavy metals
≤ 0.1 mg/L
≤ 0.1 mg/L
Chloride
≤ 3 mg/L
≤ 2 mg/L
Phosphate
≤ 0.5 mg/L
≤ 0.5 mg/L
Conductivity (at 25°C)
≤ 50 µS/cm
≤ 5 µS/cm
pH
6.5 to 8
5 to 7.5
Appearance
Clean, colorless, no sediment
Clean, colorless, no sediment
Hardness
≤ 0.1 mmol/L
≤ 0.02 mmol/L
NOTE : The values reported in this table are for information only. It is the responsibility of the user to verify the values that apply in his/her specific case.
Water for Autoclaves (Millipore n. d.)
QUALITY OF WATER FOR ENDOSCOPE WASHER-DISINFECTORS:
In 1995, the Health Technical Memorandum (HTM) 2030 was produced by the NHS Estates. It provided certain standards for the use of washer-disinfectors. It stated that:
“the final rinse water used for invasive endoscopes should be sterile, and for non-invasive endoscopes ‘it is preferable that it is sterile’.”
In an endoscopic washer-disinfector, water is required during three stages:
1. Preparation of detergent,
2. Preparation of disinfectant and
3. Final rinsing.
In order to ensure a sterile water system, there are following four methods recommended:
1. It should be filtered using pores as small as 0.1-0.22 micro meter,
2. Reverse osmosis,
3. Addition of biocides,
4. Ultraviolet light and
5. Combining different methods.
According to the Health Technical Memorandum:
“the final rinse stage should contain 1000 mV and a pH lower than 2.7. (Theme ejournals 1999)
SUPEROXIDIZED WATER:
While considering the final rinse stage, there is another type of water now introduced, known as the superoxidized water. It is basically composed of hypocholorous acid and at an approximately 144mg/L and the free chlorine radicals. The saline solution is passed over the titanium coated electrodes at 9 amp. The resultant product has a pH of about 5-6.5 with an oxidation reduction potential of >950 mV. (Medscape 2010)
Thus, the important fact for the use of water in disinfecting purposes lies in the quality of water which cannot be compromised as the ordinary water itself contains many infective organisms. Using sterile water is a standard set for both the autoclaves and the endoscope washer-disinfectors in order to ensure a high quality disinfected equipment available for use by the physician.
REFERENCES
Water for Autoclaves (n. d.) Millipore [online] available from:
http://www.millipore.com/lab_water/clw4/autoclaves
Christina Bradley (n. d.) Water Quality in Endoscope Reprocessing. Hospital Infection Research Laboratory. City Hospital Birmingham, U.K.
Decontamination and Disinfection Fact Sheet (2005) United Lincolnshire Hospitals, NHS Trust
S. Tsuji et al (1999) Endoscope Disinfection Using Acidic Electrolyte Water. Theme ejournals [online] 31(7): 528-535. Available from:
https://www.thieme-connect.com/ejournals/abstract/endoscopy/doi/10.1055/s-1999-55
Dr. Rutala and Dr. Weber (2001) New Disinfection and Sterilization Method: A New Disinfectant: Superoxidized Water. Medscape Today [online]. Available from:
http://www.medscape.com/viewarticle/414420_5
Read
More
CHECK THESE SAMPLES OF Medical Devices Decontamination
Probable Outcomes of a Radiological Dispersion Device
In a typical case, a radiological dispersion device is not used to achieve a strategic goal but, these devices are used to inflict psychological effects.... According to several studies, which have been carried out in several countries, there are several elements, which are used in the radiological devices.... The contamination caused by the radiological dispersion devices can lead to long-term health effects.... In most cases, they usually use fuels from radioactive medical waste and nuclear power plants....
6 Pages
(1500 words)
Research Paper
Methicillin-resistant Staphylococcus aureus
In the workplace, the chronic illness renal disease patients need to go to the hospital and clinic frequently; therefore, the decontamination of the environment to prevent cross transmission is most beneficial.... In the workplace, the chronic illness renal disease patients need to go to the hospital and clinic frequently; therefore, the decontamination of the environment to prevent cross transmission is most beneficial.... decontamination ensures that there are no medical or health implications in the treatment process with regard to renal medicine....
11 Pages
(2750 words)
Essay
Testing for Decontamination Devices
The annual testing is carried out on all the devices to facilitate the annual maintenance requirement (medical devices Agency).... According to medical devices Agency, the maintenance of these equipment allows for the proper functioning of the equipment during decontamination.... This saves on costs and other expenses that are incurred in purchasing new equipment (medical devices Ag... Testing for decontamination Equipment
... he choice of the level of decontamination depends on factors such as the durability of the devices, the use of the devices, and the microorganisms....
4 Pages
(1000 words)
Essay
Importance of Hand Decontamination
This paper "Importance of Hand decontamination" justifies the importance of handwashing in the clinical setting.... I have selected hand decontamination as my area of study.... Hand decontamination is an area that is a simple precautionary measure, and it is a well-known fact that nurses' hand-washing between seeing each patient would control 80% of the hospital-acquired infections, namely, dangerous methicillin-resistant Staphylococcus aureus (Royal College of Nursing, 2005)....
8 Pages
(2000 words)
Essay
First Responders Response to a Potential NBC Post Blast Incident Author
Protection of responders requires immediate decontamination of those exposed to the isolation area and their replacement with personnel wearing Personnel Protection Equipment.... rotecting first responders means immediate decontamination and medical observation for those exposed and personnel protective equipment (PPE) for those who will replace them.... Reducing the risk of contamination will require containment of the area and its perimeters to prevent unmonitored entry and exit; and evacuating and directing all persons who were in the building to the quarantine site for medical treatment, observation, and decontamination....
2 Pages
(500 words)
Essay
Sterile Service Department
After each use, the medical devices undergo various decontamination processes to render them safe and for reuse on patients and for staff handling.... After each use, the medical devices undergo various decontamination processes to render them safe and for reuse on patients and for staff handling.... However, for delicate or complex medical devices, manual cleaning is normally preferred.... Reusable medical devices are disinfected by using either a washer-disinfector at low temperature steam or by employing liquid disinfectant immersion ....
2 Pages
(500 words)
Essay
Probable Outcomes of a Radiological Dispersion Device
In a typical case, a radiological dispersion device is not used to achieve a strategic goal but, these devices are used to inflict psychological effects.... In most cases, they usually use fuels from radioactive medical waste and nuclear power plants.... The paper "Probable Outcomes of a Radiological Dispersion Device" discusses that the use of radiological weapons is considered a powerful aspect that has the capability to trigger enormous political reactions in the host countries or even in a coalition state....
6 Pages
(1500 words)
Research Paper
Medical Device Decontamination
The paper "Medical Device decontamination" is a good example of an assignment on health sciences and medicine.... decontamination is a process according to which items that need to be reused in the dental process are made safe to be used again on patients.... The paper "Medical Device decontamination in a General Dental Practice: An Audit Tool" is a good example of an assignment on health sciences and medicine.... decontamination is a process according to which items that need to be reused in the dental process are made safe to be used again on patients....
8 Pages
(2000 words)
Assignment
sponsored ads
Save Your Time for More Important Things
Let us write or edit the assignment on your topic
"Medical Devices Decontamination"
with a personal 20% discount.
GRAB THE BEST PAPER

✕
- TERMS & CONDITIONS
- PRIVACY POLICY
- COOKIES POLICY